It’s a fascinating experience to stand at Old Faithful and watch it erupt for the first time. While steam and water is vaulting into the air nearly 100 feet, it’s simply awe inspiring and beautiful to see.
There may come a time, however, when you wonder exactly how this all works. And while the specific circumstances that allow a geysers to exist are rare, it’s not a very complex issue from a physical world perspective.
Consider what a geyser is. It’s basically a spring of water coming from somewhere below the surface and traveling to the surface. Basically a column of water. Now, consider the boiling point of water. It’s 100 degrees Celsius, or 212 degrees Fahrenheit, at sea level. The “at sea level” is important, because it really means “at sea level pressure”. Anyone who has ever tried to cook a pot of beans at 10,000 feet knows that it takes a really long time. That’s because water boils at a lower temperature at higher elevations. Higher elevations means that the water is under less pressure. If you took water to space, it would at some point start to boil at room temperature. Likewise, if water decreases in elevation, the pressure increases and the boiling point rises.
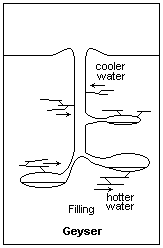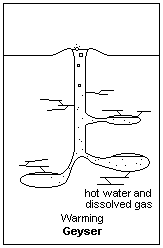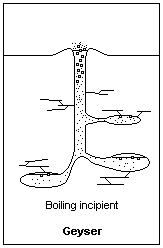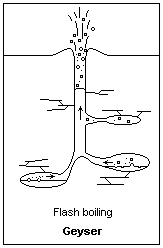
Building pressure from above allows water below to heat to higher than normal boiling Points Illustration courtesy Dr. James Calvert, University of Denver
Now consider what is happening in this underground spring that is destined to be a geyser. As you go down, the pressure on the water builds; not because of the elevation, but because of the pressure on the water from all the other water resting on top. As the pressure builds, the boiling point of the water in the bottom increases. Exactly how high it goes is not important to this part of our discussion, but suffice it to say the boiling point of water in the bottom increases to something above regular sea level boiling point. In this case, the boiling point in the bottom of the future geyser will be much higher than the boiling point of the water at the surface.
Now, introduce a geothermal feature to our equation. We’ll bring the earth’s magma closer to the geyser and heat the rocks surrounding the water column. We should probably heat it from somewhere down in the cavern because we want the bottom of the water column to heat up more than the top. Plus, we want the water that’s been heated in the bottom to rise to the top. Remember that hot air rises and cool air descends. So does hot water compared to cooler water.
As the water in the bottom of the water column starts to heat, it starts to rise. Eventually, the water in the bottom reaches a point that it’s above the boiling point that water would be at if it were at the surface. That’s because it’s at higher pressure than the water at the surface. Let’s say that the water in the bottom has reached a point that, if it were at the surface, it would be way above the boiling point, and be converted to steam. As the water starts to rise, that’s exactly what happens. The rising water is exposed to less and less pressure as it goes higher in the column and eventually reaches a point where it starts to boil. When it boils, air bubbles appear in the water which displaces some of the water volume. The only place for it to go out the top of the geyser. As the water ejects from the top, it relieves pressure from down below and the water that was down deeper flashes into steam.
A geyser erupts.










[…] By the way, we spent some time a few years ago learning how a geyser works; what makes it erupt. We then wrote up a post that explains the process the best we could. If you ever wondered what was involved in the mechanics of an erupting geyser, take a look at this post, entitled, “Making a Geyser.” […]